Product sourcing simplified
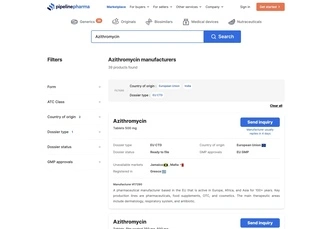
Search the largest EU CTD database of finished-dose formulations today for human use. Discover generics, biosimilars, medical devices and nutraceuticals.
Have more advanced needs? We are ready to help you find the most attractive products for your market or develop them on your behalf.
-
Get vetted information on products and manufacturers
-
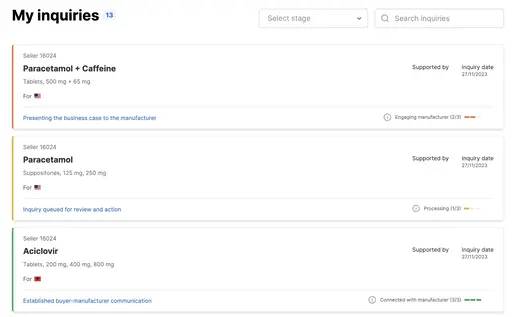
Make requests for quotations easily
-
Use deal structuring and deal facilitation support
-
Connect with manufacturers directly
Product sourcing simplified